English
English
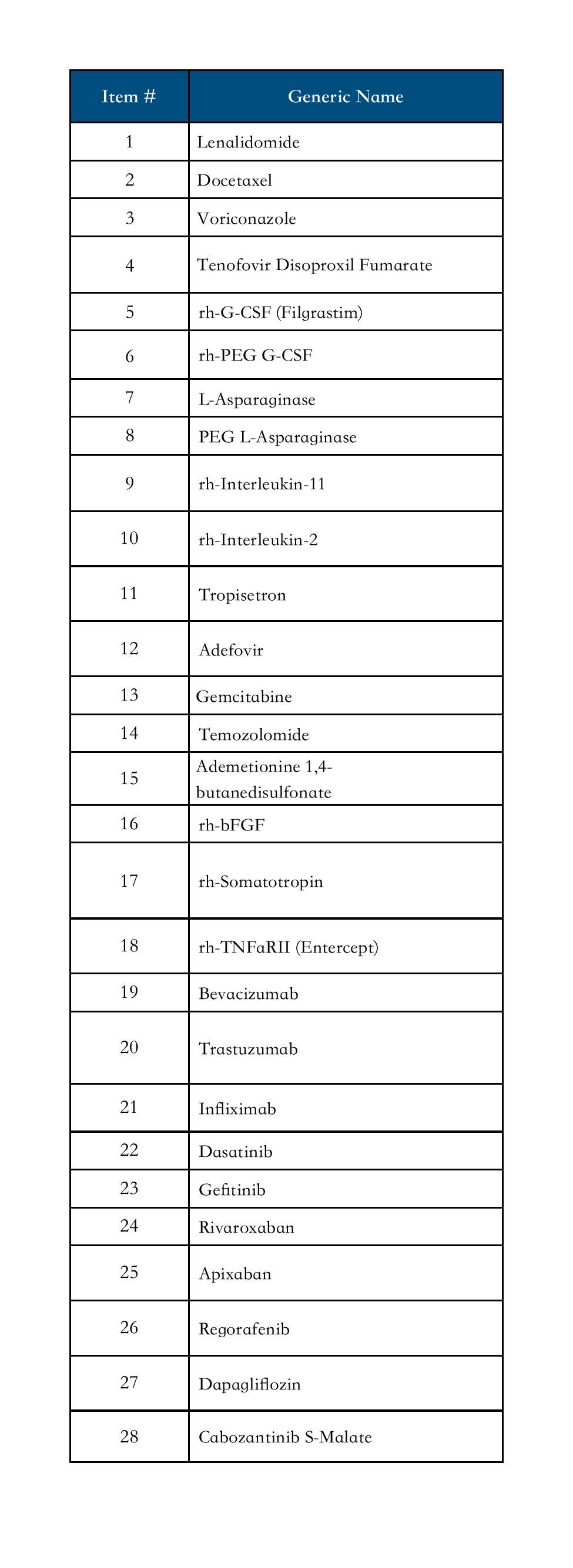
Finished Formulations
Temozolomide; Arsenic Trioxide; Lenalidomide; Docetaxel; Paclitaxel; Filgrastim (Recombinant Human Granulocyte Colony-Stimulating Factor, rh-G-CSF); Recombinant Human Interleukin-11 (rh-IL-11); Recombinant Human Interleukin-2 (125Ala IL-2); Thymopentin; Vinorelbine Bitartrate; Hydroxyl-camptothecine; Nimustine Hydrochloride; Asparaginase; PEG L-Asparaginase; Calcium Foliate; Recombinant Human Basic Fibroblast Growth Factor (rh-bFGF); Liraglutide; Insulin Aspart 30; Insulin Aspart 50; Insulin Aspart; Recombinant Human Follitropin(rh-FSH); Ciclosporin; Mycophenolate Mofetil Dispersible; Ornidazole; Voriconazole; Adefovir Dipivoxil; Levofloxacin Hydrochloride; Azithromycin; Ribavirin; Tenofovir Disoproxil Fumarate; Valacylovir Hydrochloride; Lysozyme; Stavudine; Tropisetron Hydrochloride; Granisetron Hydrochloride; Omeprazole; Ademetionine 1,4-Butanedisulfonate; Glutathione; Rivaroxaban; Enoxaparin Sodium; Coenzyme Complex; Ginkgo Biloba Extract; Simvastatin; Telmisartan; Naftopidil; Nicergoline; Zaleplon; Cytidine Disodium Triphosphate; Febuxostat; Risedronate Sodium; Loratadine Dispersible; Somatostatin; Octreotide Acetate; Calcitonin (Salmon); Paracetamol and Tramadol Hydrochloride; Etodolac
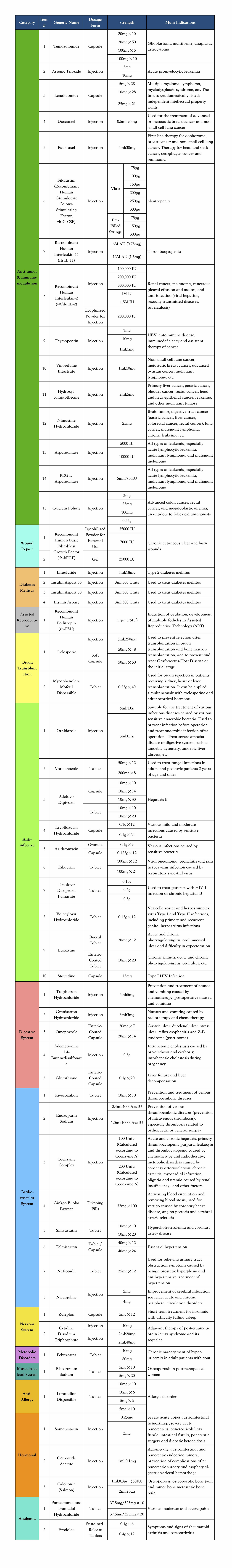
Active Pharmaceutical Ingredients (API)